



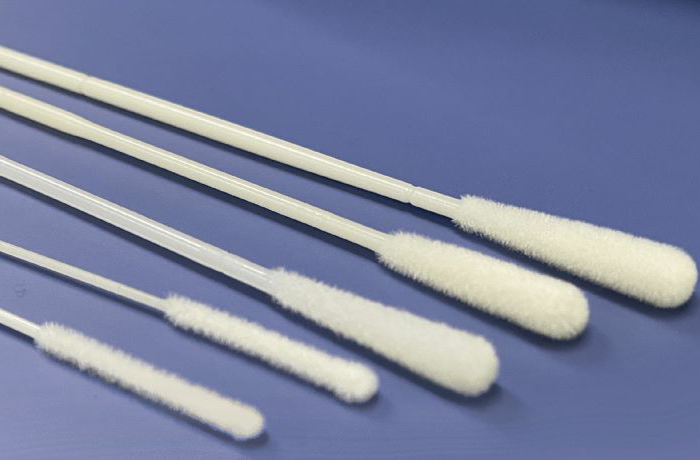
En comparación con los hisopos tradicionales, tiene mejores capacidades de adsorción y elución de muestras, adecuado para la recolección de muestras de secreciones, virus o células.
Vida útil: 3 años.
Condiciones de almacenamiento: 2-30 ℃
La cabeza del hisopo está firmemente adherida, tiene un tamaño uniforme y no tiene puntos negros. La varilla de plástico es suave sin rebabas. Sin partículas duras.
Para obtener más información, consulte nuestras instrucciones de uso.
For nasopharyngeal swab, the molded break point are: 8cm and 10cm
For oropharyngeal swab, the molded break point are: 3cm, 8cm and 3&8cm.
If the quantity is considerable, we accept customization.
Operators who use this product should be trained and wear mask, gloves and other relevant protective equipment.
During use, do not touch the swab head with hands or other objects.
The product is only used for sample collection, for one-time use only and cannot be reused.
Never use the product when its package is damaged.
Never use the product when it expires.
The collected samples must be sent for inspection as soon as possible to ensure the accuracy of the results;
The risk of choking! Be careful when the sampling swab is inserted into the mouth, throat, or nasal cavity.
The risk of breakage! Do not use violent force during the swab sampling process to prevent the swab from breaking.
Ensure that the top of the sampling swab does not touch any surface before collection;
The samples used and their culture wastes should be regarded as potentially infectious substances, and should be handled in accordance with the operating specifications of the infectious disease laboratory before they can be discarded;
When collecting samples, professionals should strictly follow the national sampling procedures; when samples are tested, they should be operated in laboratories that meet the safety level.
The product should be stored in a dry, ventilated, non-radiation and no corrosive gas clean room.
If there is any serious incident occurs in relation to the device, please report to the manufacturer and the competent authority in your country immediately.