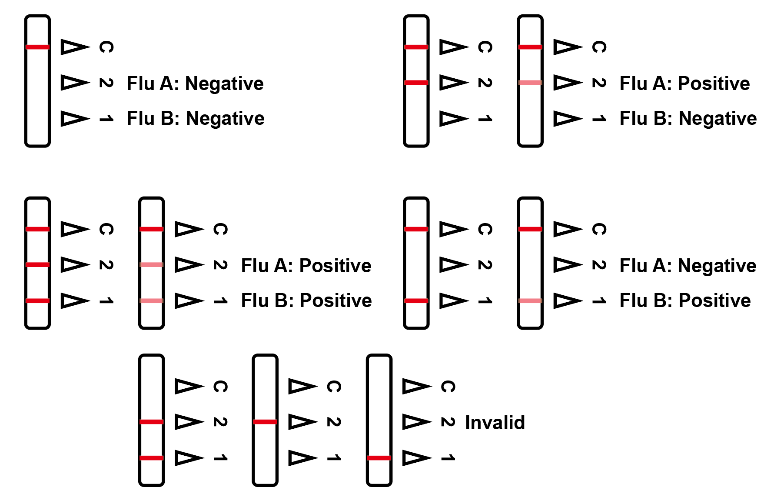
The Influenza A&B Rapid Test Cassette is a qualitative, lateral flow immunoassay for the detection of Influenza A and Influenza B nucleoproteins in nasal swab specimens. In this test, antibody specific to the Influenza A and Influenza B nucleoproteins is separately coated on the test line regions of the test strip. During testing, the extracted specimen reacts with the antibody to Influenza A and/or Influenza B that are coated onto particles.
The test kit can be stored at room temperature or refrigerated (2-30°C) for the duration of the shelf life.
Clinical Sensitivity, Specificity and Accuracy
The Influenza A&B Rapid Test Cassette has been evaluated with specimens obtained from the patients. RT-PCR is used as the reference method for the Influenza A&B Rapid Test Strip (sterile swab). Specimens were considered positive if RT-PCR indicated a positive result. Specimens were considered negative if RT-PCR indicated a negative result.
Influenza A:
Sensitivity: >99% (89.4~100%)*
Specificity: >99% (94.6~100%)*
Accuracy: >99% (96.4~100% )*
*95% Confidence
Influenza B:
Sensitivity: >99% (88.8~100% )*
Specificity: 98.6% (92.2~99.9% )*
Accuracy: 99.0% (94.6~99.9%)*
*95% Confidence
Subtypes of Influenza A:
A/NWS/33 10(H1N1), A/Hong Kong/8/68(H3N2), A/Port Chalmers/1/73(H3N2), A/WS/33(H1N1),A/New Jersey/8/76(HswN1),A/Mal/302/54(H1N1),A/Cambodia/RO405050/2007(H5N1),A/Vietnam/1194/2004(H5N1) and A/Hong Kong/1073/99(H9N2)
Subtypes of Influenza B:
Bright, B/R5, B/Russia/69, B/Lee/40 and B/Hong Kong/5/72