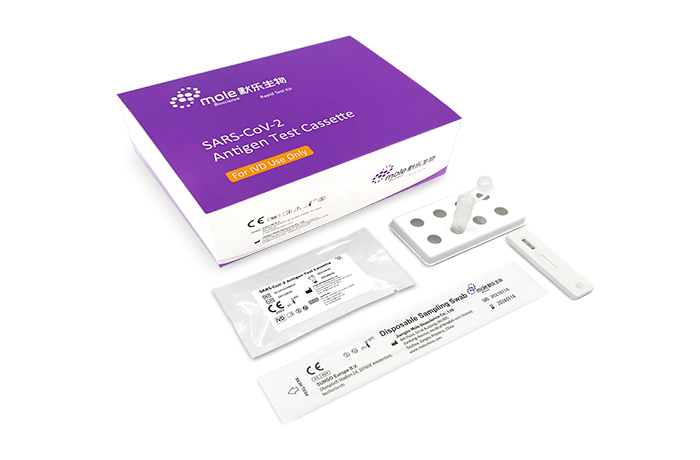
The SARS-COV-2 Antigen Rapid Test is a qualitative detection of SARS-COV-2 virus in nasal swab or saliva specimens, the virus can be detected within the first week of the onset of symptoms. The test strip is composed of the following parts: sample pad, reagent pad, reaction membrane, and absorbing pad. The reagent pad contains the colloidal-gold conjugated with the monoclonal antibodies against the nucleocapsid protein of SARS-CoV-2. The reaction membrane contains the secondary antibodies for nucleocapsid protein of SARS-CoV-2. The whole strip is fixed inside a plastic device.
Store at 4-30°C in the sealed pouch until the expiration date printed on the package. Do not freeze.
1. Sensitivity, Specificity and accuracy
The SARS-CoV-2 Antigen Test Cassette was compared with a commercially available gold standard reagent (PCR), the results showed that the clinical sensitivity and specificity.
Nasopharyngeal Swab:
Clinical Sensitivity (Positive coincidence rate):98.31% (95% CI: 94.00% ~99.50%)
Clinical Specificity (Negative coincidence rate):99.17% (95% CI: 95.50%~99.90%)
Nasal Swab:
Clinical Sensitivity (Positive coincidence rate):97.30% (95% CI: 93.30% ~99.90%)
Clinical Specificity (Negative coincidence rate):99.19% (95% CI: 96.00%~99.90%)
Saliva Specimen:
Clinical sensitivity (Positive coincidence rate):94.31% (95%CI: 88.72%~97.22%)
Clinical specificity (Negative coincidence rate):100.00% (95%CI: 96.90%~100.00%)
German Instruction for use link:
![]() DE_R220_BMA5680501_IFU_SARS-CoV-2 Antigen Test Cassette_Ver1.0_For Professional Use_20211208.pdf
DE_R220_BMA5680501_IFU_SARS-CoV-2 Antigen Test Cassette_Ver1.0_For Professional Use_20211208.pdf
Performance and stability report:
![]() SARS-Cov-2 Antigen Test Cassette_Stability Report.pdf
SARS-Cov-2 Antigen Test Cassette_Stability Report.pdf
![]() SARS-Cov-2 Performance Study Report.pdf
SARS-Cov-2 Performance Study Report.pdf
Fast detection which can getting the test result in 15 minutes.
This test is easy to use and does not require laboratory equipment.
Cost-efficient and can be done at the point of care.
Allowing decentralized testing or access to area where laboratory testing is not available.